Table of Contents
Introduction
In an age where biological innovation thrives on precision, the preservation of cellular materials, tissues, and nucleic acids demands uncompromising fidelity. Cryogenic vials, though diminutive in form, play a pivotal role in securing the long-term viability and integrity of valuable biospecimens. Whether in clinical repositories, academic laboratories, or pharmaceutical biobanks, cryogenic storage is a linchpin of modern bioscience.

Table of Contents
Understanding Cryogenic Vials
A cryogenic vial is a laboratory-grade container specifically designed to withstand ultra-low temperatures, typically ranging from –80°C down to –196°C. These vials enable the cryopreservation of cellular material, ensuring biological activity is paused rather than degraded. From rudimentary ampules in early cryobiology to today’s rigorously engineered vessels, cryogenic vials have evolved in tandem with advancements in storage methodology.
Material Science of Cryogenic Vials
Modern cryogenic vials are predominantly constructed from high-purity polypropylene. This thermoplastic polymer offers the chemical inertness and mechanical resilience needed to survive immersion in liquid nitrogen without shattering. Additive-free formulations are often preferred to minimize potential leachables, safeguarding sample purity and compatibility with sensitive assays.
Structural Design Features
Design differentiation enhances utility and compatibility. External-threaded caps minimize contamination risk, while internal-threaded variants reduce rack space usage. Wall thickness ensures the vessel withstands internal pressure buildup during thawing. Self-standing bases provide stability in racks, while round-bottom options are optimized for cryobox storage density.
Cap and Seal Integrity
The cap design is paramount. Screw-threaded systems incorporate elastomeric O-rings or silicone gaskets to achieve leak-proof, vapor-tight closure. These seals prevent both ingress of contaminants and egress of volatile sample contents, even during thermal stress. Rigorous engineering ensures that cap retention force and seal compression are finely tuned.

Volume and Dimension Options
Cryogenic vials are available in standard volumes such as 0.5ml, 1.5ml, 2.0ml, and 5.0ml. The vial’s height and diameter must align with the intended cryostorage rack, especially when space efficiency is critical. Uniform dimensions simplify robotic handling in automated biobanking systems.
Sterility and Contamination Control
Manufacturers ensure sterility via gamma irradiation or e-beam sterilization. Certified DNase-, RNase-, and endotoxin-free status affirms the absence of nucleic acid-degrading enzymes and microbial pyrogens. These standards are especially vital for genomic and proteomic workflows.
Labeling and Identification
Durable identification is essential. Cryogenic vials are equipped with writable, frost-resistant labeling areas or pre-applied barcode labels. Advanced models feature laser-etched datamatrix codes compatible with LIMS (Laboratory Information Management Systems), enabling seamless sample tracking.
Cryogenic Storage Methods
Storage modalities influence vial selection. In liquid phase nitrogen (–196°C), vials must resist both thermal shock and liquid ingress. Vapor phase storage, while slightly warmer, reduces contamination risk. Racking orientation—vertical or horizontal—must accommodate vial design and retrieval frequency.
Temperature Resilience and Stability
The thermal profile of a cryogenic vial must remain stable over countless freeze-thaw cycles. Vials are tested for crack resistance at cryogenic thresholds, ensuring they maintain structural integrity despite rapid thermal transitions.
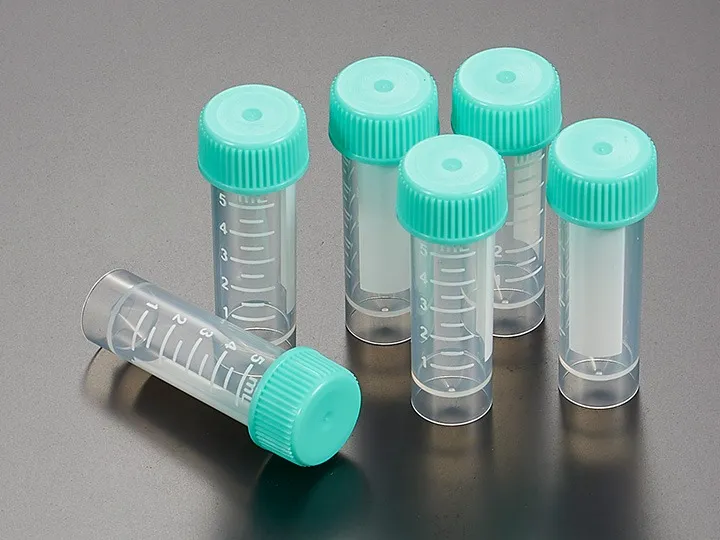
Leakage and Safety Mechanisms
Triple-seal designs, with secondary and tertiary barriers, offer redundancy against leakage. Pressure-release valves or venting notches mitigate rupture risks during warming, a frequent failure point in inferior vials.
Applications in Clinical and Research Settings
Cryogenic vials are indispensable in the storage of patient-derived cells, stem cell lines, tumor biopsies, and nucleic acids. Their application spans regenerative medicine, clinical diagnostics, pharmacogenomics, and personalized therapy.
Compliance and Certification Standards
High-quality cryogenic vials bear certifications such as CE marking, ISO 13485 for medical device quality, and USP Class VI for biocompatibility. These credentials confirm suitability for clinical and translational research use.
Biobanking and Global Sample Logistics
From local collection to international shipment, vials must endure vibration, temperature fluctuations, and handling variability. Secure capping and validated container integrity uphold sample fidelity throughout the cold chain.
Innovations in Cryogenic Vial Technology
Contemporary advances include color-coded caps for visual workflow segregation and pre-barcoded vials to eliminate labeling errors. Some models are integrated with RFID chips for real-time inventory control.
Environmental Considerations
Eco-conscious labs now opt for vials made from recyclable or bio-based polymers. Initiatives to reduce single-use plastic volume in cryostorage are gaining traction, promoting both sustainability and performance.
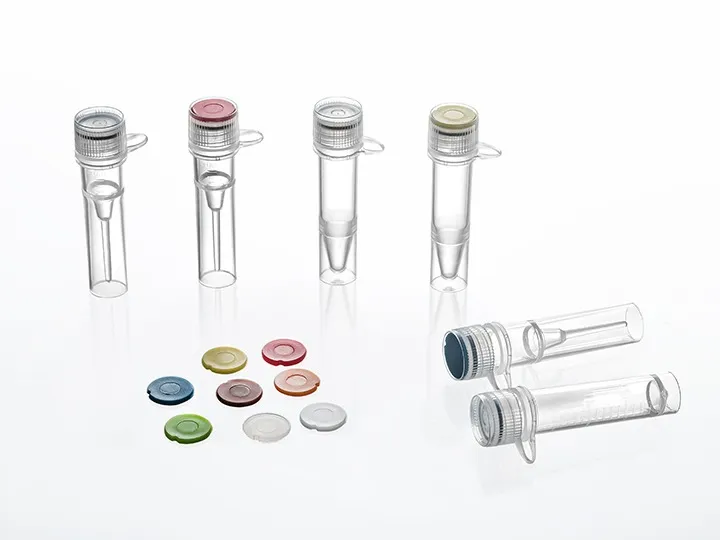
Choosing the Right Cryogenic Vial
Lab-specific requirements—sample type, storage duration, retrieval frequency—dictate vial choice. Reliable suppliers provide technical data sheets, lot certification, and sample retention data to guide procurement decisions.
Training and Handling Protocols
Proper use of cryogenic vials requires training. Personnel must be adept in working with LN2, using PPE, and following SOPs for loading and unloading samples under sterile conditions.
Maintenance and Inventory Management
Routine audits of stored samples, including inspection for label legibility, cap integrity, and storage date compliance, ensure sample traceability and prevent data loss. Digital inventory systems enhance oversight.
Common Challenges and How to Avoid Them
Avoid sudden immersion of warm vials into LN2 to prevent cracking. Use appropriate cryo-labels to avert identity loss. Regularly test vials for cap loosening and structural fatigue.
Case Studies and Performance Benchmarks
Independent studies show that triple-sealed, externally threaded cryovials outperform generic models in leak prevention. Labs report higher post-thaw viability with premium-certified vials.

Conclusion
Cryogenic vials are more than simple containers; they are engineered instruments of preservation. As biobanking, cell therapy, and genomic research advance, the demand for resilient, compliant, and sustainable cryogenic vial solutions will only intensify. Their role in safeguarding biological heritage is indispensable, ensuring that every frozen sample remains a viable asset for scientific discovery.
